Matrix OI® FLEX IT
Providing the next generation
of regenerative therapies.

Call us today at
(877) 887-3436
We are a distributor of amnion-derived products and other medical solutions to physicians, surgeons, clinics, and hospitals.
Matrix OI® FLEX IT
Matrix OI® FlexIT, when hydrated, is a thin pliable cortical sheet that has the ability to be sized with scissors or a scalpel.
Matrix OI® products are verified for osteoinductivity prior to release for distribution. In-vivo Matrix OI® test results demonstrate all five (5) bone-forming elements present (Chondrocytes, Osteocytes, Bone Marrow, Cartilage, and New Bone). In-vitro Matrix OI® test results for BMP levels demonstrate CellRight’s Matrix OI® products have up to 19x the native BMP levels of the BMP-2 control.
The grafts are freeze-dried and sterilized using low-dose gamma irradiation to achieve a sterility assurance level (SAL) of 10-6. The grafts should be stored in ambient temperatures (59-86o F or 15-30o C) and have a shelf life of five-years from the date of packaging.
CellRight’s proprietary next-generation BioRinseTM processing technology has been proven to preserve native bone morphogenic proteins (BMP’s).
Matrix OI® FlexIT is indicated for homologous use in craniomaxillofacial applications including cranial repair, orbital floor, and zygomatic fractures, involving sutures, plates, anchors, and other fixation devices. Other uses for Matrix OI® FlexIT include reconstruction, posterolateral spinal procedures, long-bone fracture plate, non-unions, and dental procedures.
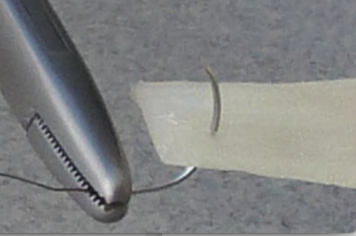
Features and Benefits
- 100% bone
- Osteoinductive – each lot is in-vivo tested
- Osteoconductive
- Aseptically processed
- Sterile – sterility assurance level of 10-6
- Five-year shelf life
- Ambient storage and shipping temperatures
- Resists migration
- Available in multiple geometries and sizes
Call us today at
(877) 887-3436
REGENISOURCE PROVIDES THE NEXT GENERATION OF REGENERATIVE THERAPIES.
TESTIMONIALS
“My knee injections with amnio did exceptionally well for me, even letting me return in to competitive cycling. I put 1000 miles on my racing bike last year, only 6 months after my amnio injections.”
“In my 7 years using human placental tissue allografts patients experienced a more rapid recovery time than conventional standard of care treatments. Patients and I noted a significant decrease in inflammation/ swelling which reduces pain and increases function. Unlike cortisone which has a long list of side effects, no adverse events have ever been reported using these placental tissue allograft.”

